The publication of the new EU fertilizers regulation in 2022 has brought new opportunities to the European market. So far, Van Iperen International has achieved eight products officially certified.

In July 2022, the EU Fertiliser Products Regulation (EU) 2019/1009 became effective. And with it, a new regulatory framework and rules entered the European fertilizer market. Are you familiar with the new EU fertilizing product regulation? To understand better consequences on growers, distributors, and manufacturers, we talk to Tove van der Putten, Head of Regulatory Affairs at Van Iperen International.
Since the announcement of the new European fertilizer law in 2019, Tove has been handling its implementation. Thanks to her team efforts, today, Van Iperen International has the official certificate for 8 products… and counting! “We are one of the firsts to achieve the certification for biostimulants and, definitely, one of the firsts in Europe with such an extensive range of products”, says Tove.
Question 1 (Q1): What consequences bring the implementation of the EU Fertiliser Products Regulation (EU) 2019/1009 to growers? And what’s the impact on the distributors?
Tove: Thanks to the FPR (EU) 2019/1009, growers will now have easier access to a wider range of high-quality efficient fertilizers and biostimulants in the European market. Also, they will get more information on how to use and not overuse the products. But in many ways, they will not be affected other than that the product labels will have more info than before.
As far as the chain of users (where distributors play an important part), we all have a certain responsibility in the process. Van Iperen International, as the manufacturer, is the main responsible party for upholding the assessment of each product. However, it is key that the distributors are involved and make sure the manufacturers do their job.

Q2: What are the main differences between the EU Fertilizer Products Regulation (EU) 2019/1009 and the previous regulation?
Tove: For the market, it is a huge difference between minerals and organic products.
- For the mineral products, it is adding more control over raw materials and the amount and quality of the fertilizer added to the European soil.
- For organic products and biostimulants, it is giving the option of free movement within the whole EU, which has not been an option before now.
Compared to the previous law, the introduction of the FPR guarantees rigorous controls over the claims of the certified products, besides providing the grower with reliable information about the production process. However, the workload for our Regulatory department has definitely increased, and it requires more out of us than ever before. As part of the Regulatory Team, the workload has definitely increased, and it requires more out of us than ever before.
Q3: What new opportunities bring this regulation to Van Iperen and the fertilizer sector? What are the challenges?
Tove: The opportunities lie in organic products and biostimulants. Although the biostimulation assessment process is not an easy one, the fact that it reaches all EU countries in one go is a big change from the local registrations and mutual recognition. The challenges lie in changing an entire market that is in many aspects set in its ways.
This new EU Fertilizing Product law requires a lot more knowledge and control over the production and quality control of our fertilizers. But it is good, and in my opinion, a crucial step in making the business greener, more sustainable, and safer for our environment.
Q4: What steps is the sector demanding to regulate?
Tove: One aspect which is still not finalized and published is the standard that will be used to make the analytic and agronomical assessment of all products. Until that happens we follow ISO and GLP standards. What is most important in the end is that the methods used are reproducible and explainable.
Q5: The new Fertilizing products Regulation requires a product assessment. What does it consist of?
Tove: We assess each product on two main criteria:
- Firstly, if the raw material and production process are within the allowed options.
- Secondly, the minimum nutrient and maximum impurities need to be safeguarded.
With agronomical information, they make up the technical dossiers of each product. The proof will be the declaration of conformity and, in the end, the CE mark placed on each label.
Q6: Could you describe the registration process and summarize the steps taken till completing it? What’s the role of the certification bodies?
Tove: Since the biostimulants have specific agronomical claims on crops, these products need external approval. For it, Van Iperen International has undergone an external audit process with the cooperation of the Dutch certification body KIWA. This process has consisted of a full company and production site audit, resulting in the official certificate for our biostimulant range.
This process ensures that each claim is based on accurate data and, subsequently, that the grower is not misled when buying a CE-marked biostimulant. For Van Iperen, it is a welcomed step to legitimize our products.
Q7: When did you start working on the implementation of the new regulation at Van Iperen?
Tove: The first briefings from the working meetings of the EU commissioner were already discussed within our Regulatory department in 2017. In June 2019, the law was finally published. The same year the communication with the Dutch agricultural ministry slowly started. Finally, in the spring of 2021, cooperation with KIWA began. We did a pre-audit together with them in order to help them receive their accreditation from the Dutch agricultural ministry and the EU commission. They became the third certification body to get accredited in the EU in January 2022. After two audits in April and October, we’ve received our certification in December 2022. From this point, the focus will be on our internal assessment process in order to cover as much of our portfolio as possible.
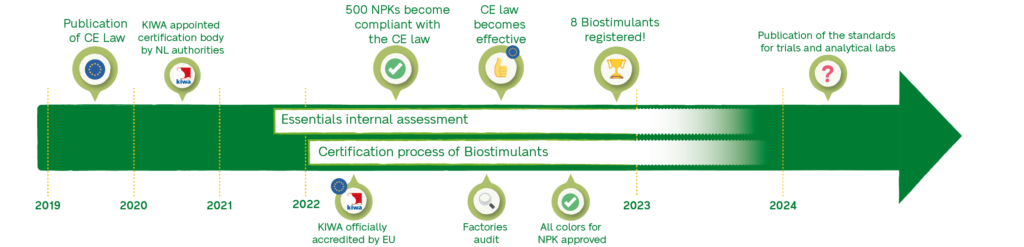
Q8: Regarding product registration, what’s the current status of Van Iperen products? What blocking points did you encounter? What’s been the easiest to sort out?
Tove: For the non-biostimulant products, we do an internal assessment. This process is currently ongoing, and we work our way through our extensive product portfolio. In some instances, we also are struggling with finding the correct method for specific parameters and product characteristics.
For the biostimulants, the biggest bottleneck has been that we were one of the first companies asking for such an audit. When you are the first, the road is not always straightforward.
Q9: The FPR (EU) 2019/1009 opens the door to agronomical claims on products. Could you explain it through a real example of Van Iperen products?
Tove: Before the FPR entered into force our biostimulation products depended on local registrations and mutual recognition. And even then, not all EU countries had the option to register the products as full biostimulants. Since July 2022, we not only have the option to freely market and sell in all of the EU but also reach the full status of biostimulant that our High Performing Solutions.
With the implementation of the EU Fertilizer Products Regulation, Van Iperen adds an extra asset to its vast product catalogue. This law sets new rules to guarantee quality and trust for growers looking for enduring solutions. And Van Iperen will continue working to assure the European standards on all solutions.